SOLVED: Part V: Solubility; Like dissolves In Like: Search online for the solubility of the above solvents in water. List all solvents that are miscible [ in water from Part IV: Emanol,Ubfr ,

Conventional Miscibility Test Therefore, Simulation method should be... | Download Scientific Diagram
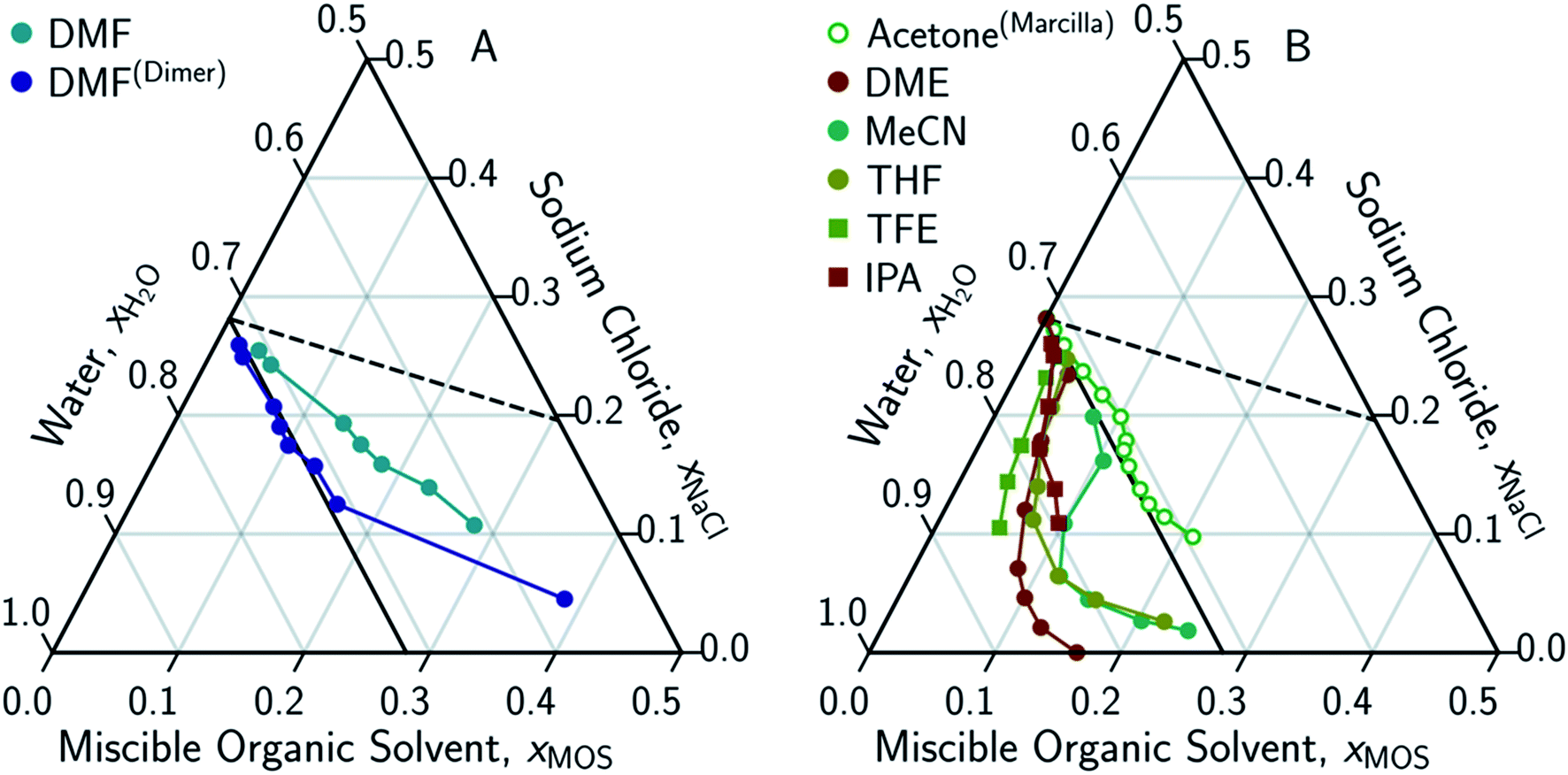
Solute displacement in the aqueous phase of water–NaCl–organic ternary mixtures relevant to solvent-driven water treatment - RSC Advances (RSC Publishing) DOI:10.1039/D0RA06361D
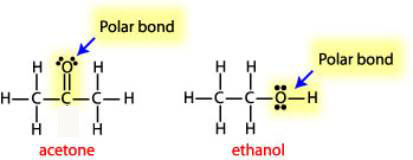
Solvent Polarity and Miscibility How are the relative polarities calcualted? Also, why is methanol less polar than ethanol? (Refer to link highlighted in blue please). - Quora
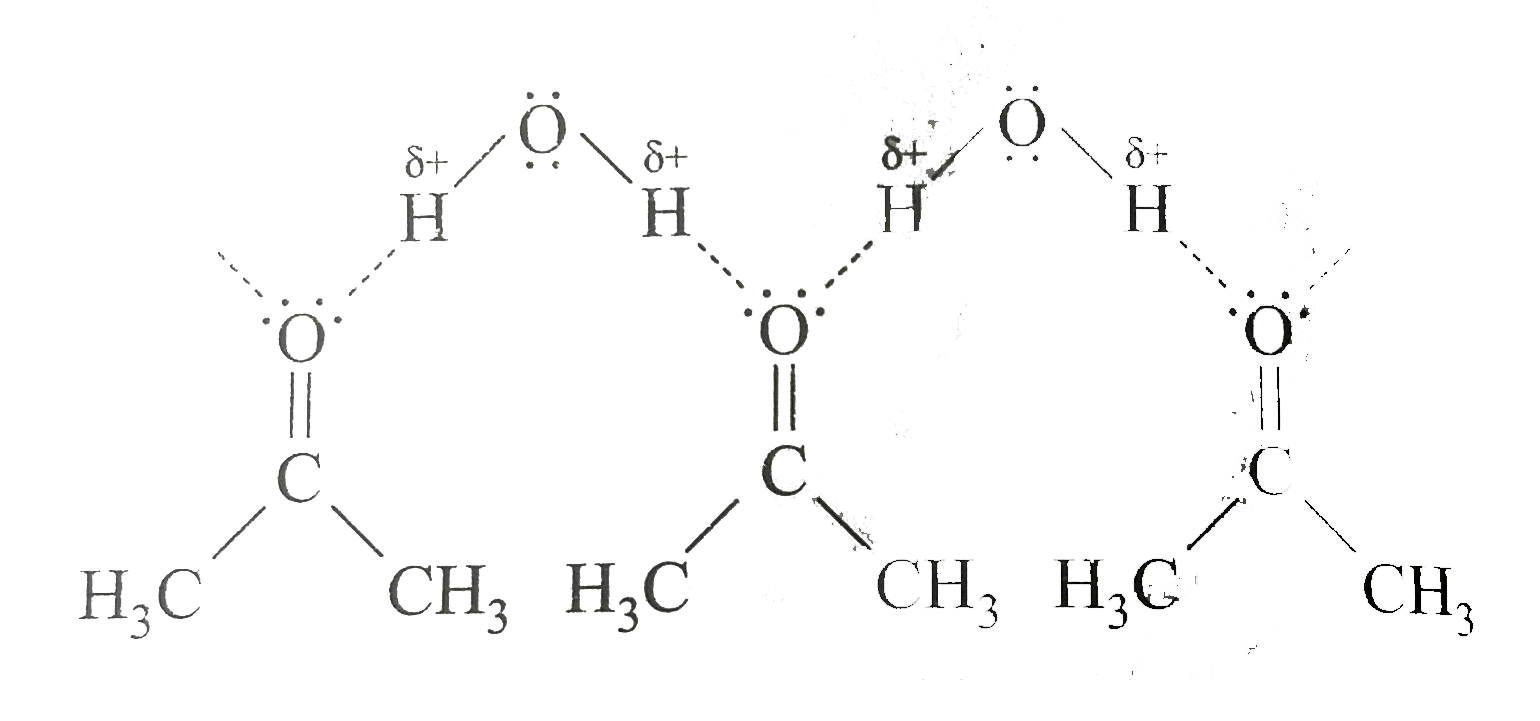
Which of the following is correct I Water and octane are essentially in one another II Water acetone are miscible III LiCl is highly soluble in water, less solube in acetone and

Acetone, or propanone, is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone. It is … | Chemical structure, Chemistry, Molar mass

Solute displacement in the aqueous phase of water–NaCl–organic ternary mixtures relevant to solvent-driven water treatment - RSC Advances (RSC Publishing)

Which of the following is correct I Water and octane are essentially in one another II Water acetone are miscible III LiCl is highly soluble in water, less solube in acetone and