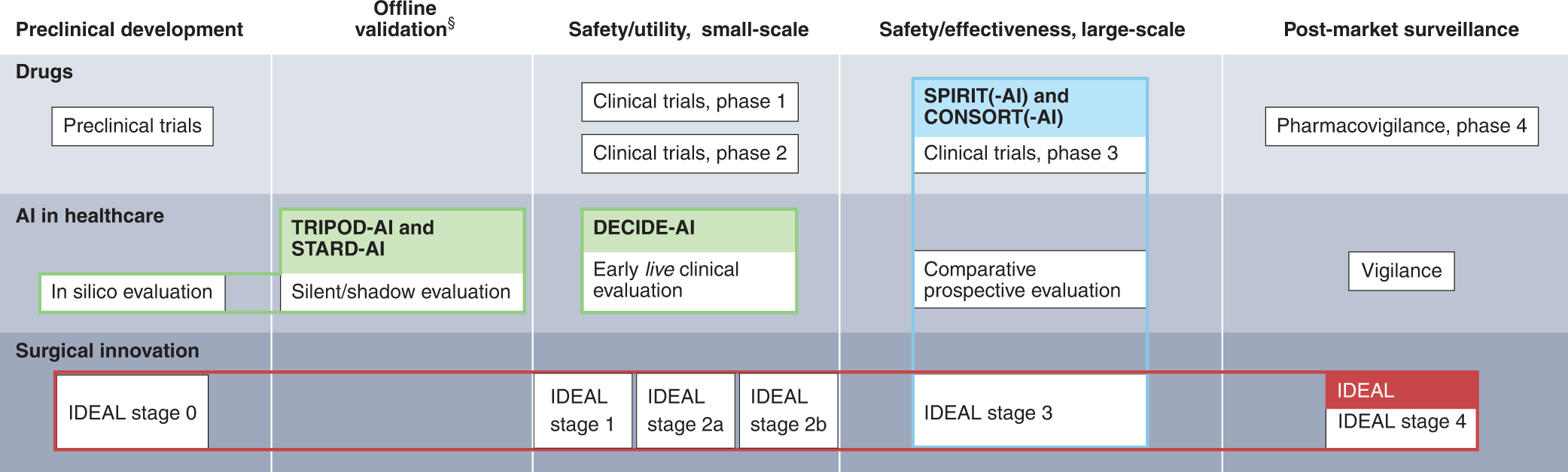
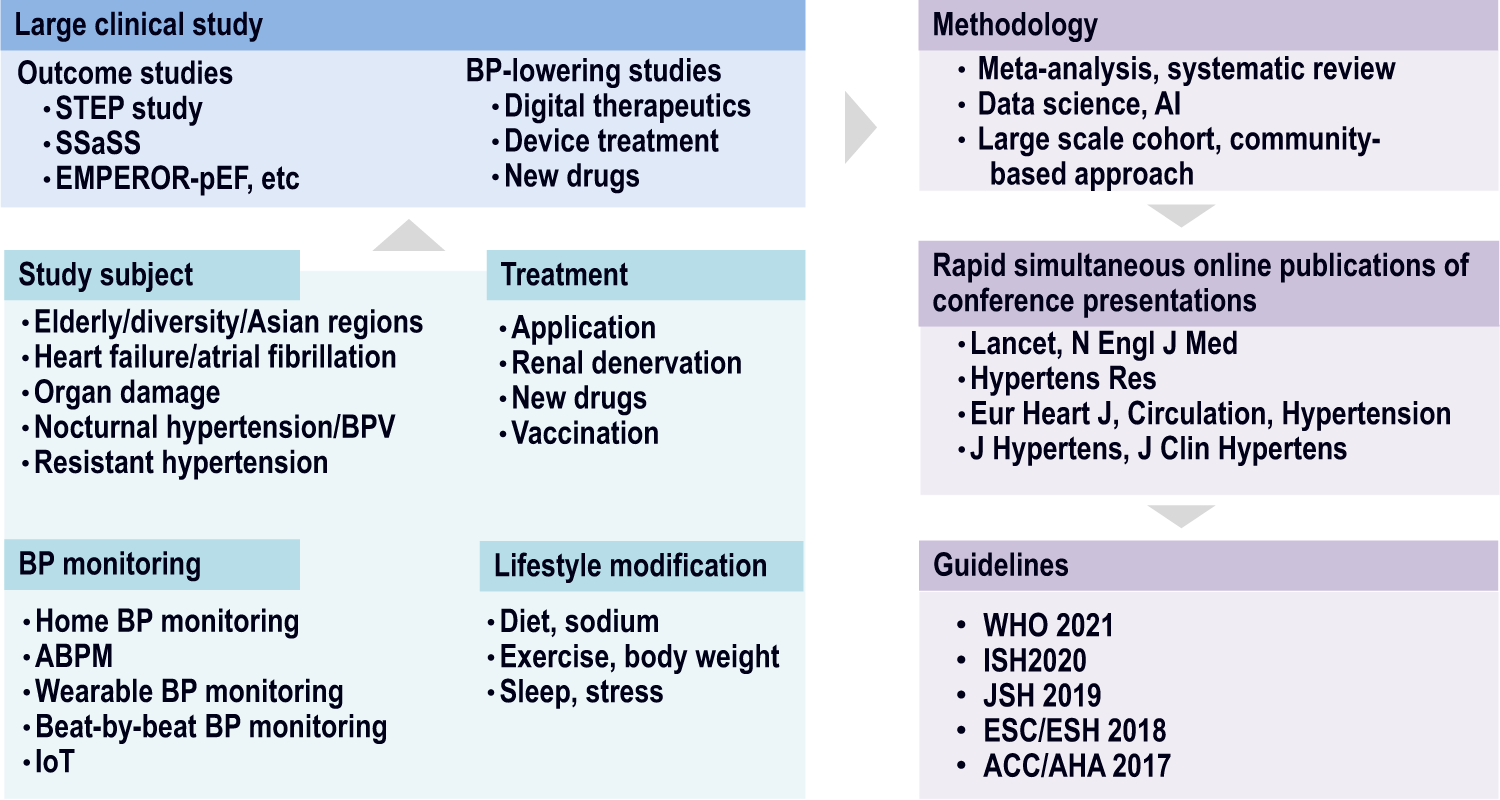
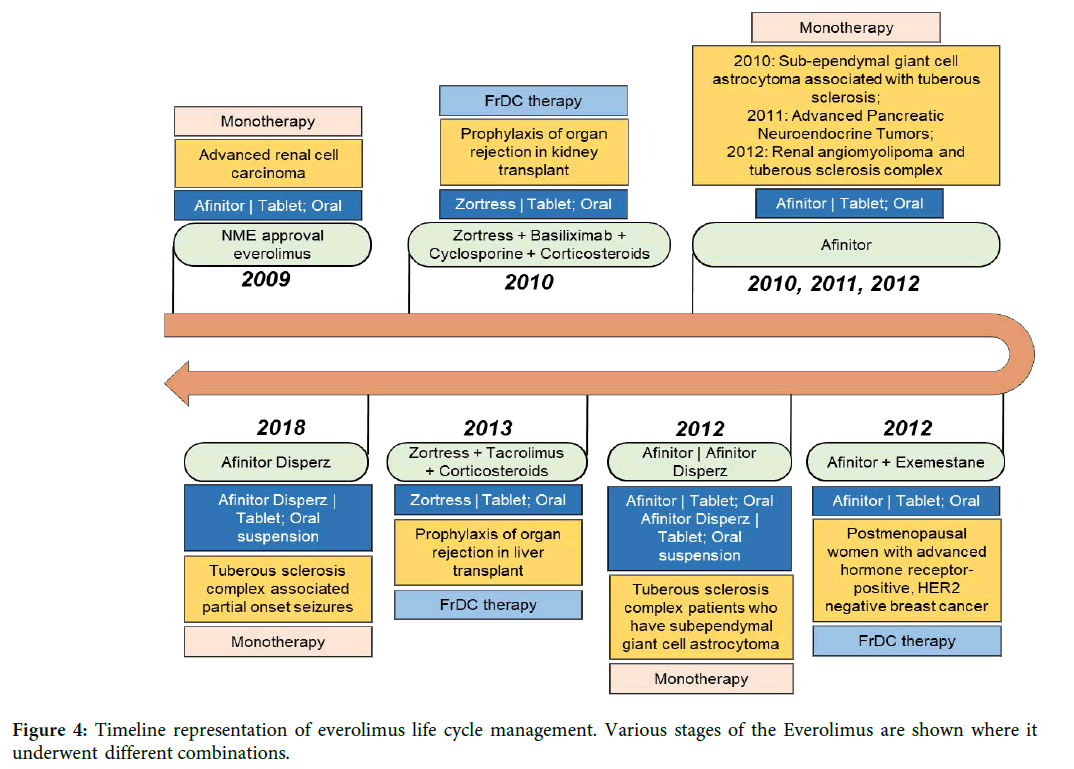
Full article: Fixed dose drug combinations – are they pharmacoeconomically sound? Findings and implications especially for lower- and middle-income countries

Pharmaceutical application and development of fixed-dose combination: dosage form review | SpringerLink

Regulatory guidelines and preclinical tools to study the biodistribution of RNA therapeutics - ScienceDirect

Fixed-Dose Combinations Improve Medication Compliance: A Meta-Analysis - The American Journal of Medicine

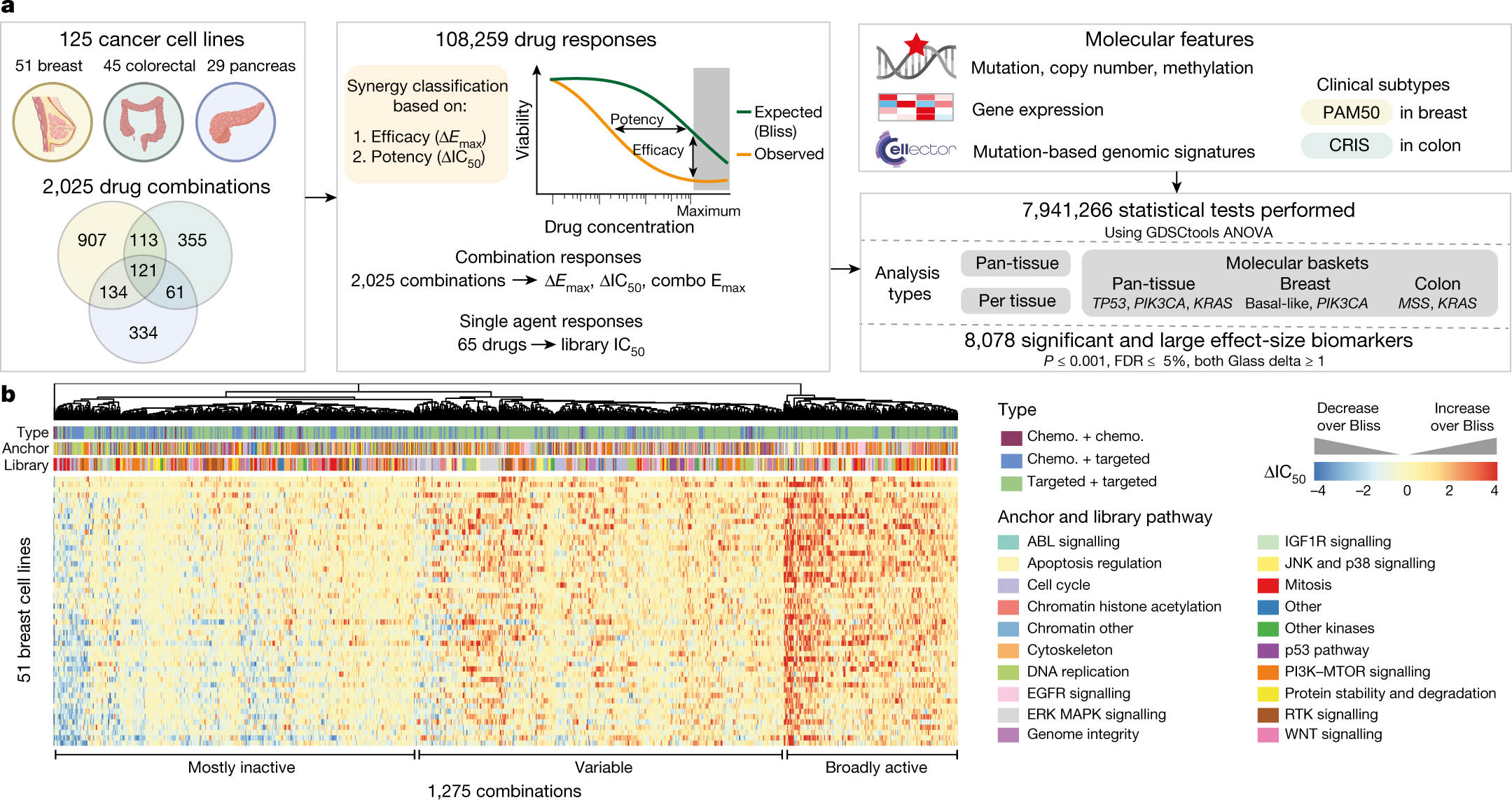
Evaluation of synergism in drug combinations and reference models for future orientations in oncology - ScienceDirect

Challenges and opportunities to include patient‐centric product design in industrial medicines development to improve therapeutic goals - Timpe - 2020 - British Journal of Clinical Pharmacology - Wiley Online Library

Annex 5 Guidelines for registration of fixed-dose combination medicinal products - PDF Free Download
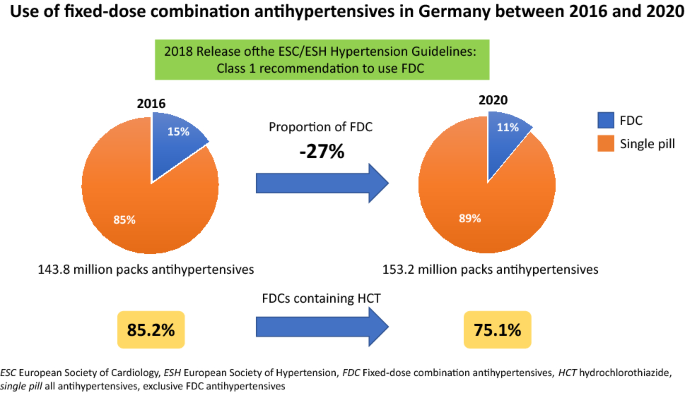
Use of fixed-dose combination antihypertensives in Germany between 2016 and 2020: an example of guideline inertia | SpringerLink

Initial treatment with a single pill containing quadruple combination of quarter doses of blood pressure medicines versus standard dose monotherapy in patients with hypertension (QUARTET): a phase 3, randomised, double-blind, active-controlled trial -
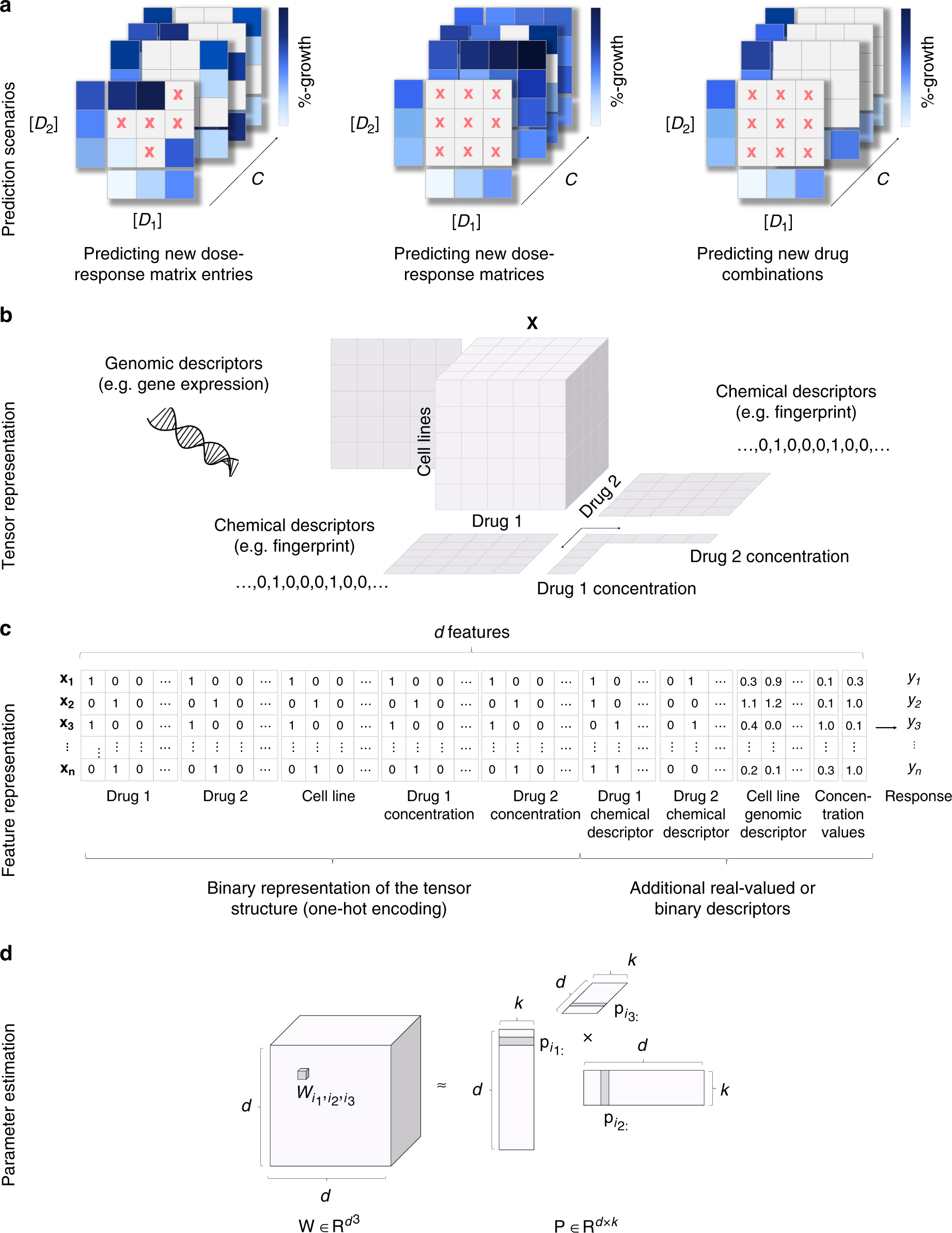
Leveraging multi-way interactions for systematic prediction of pre-clinical drug combination effects | Nature Communications

Pharmaceutical application and development of fixed-dose combination: dosage form review | SpringerLink