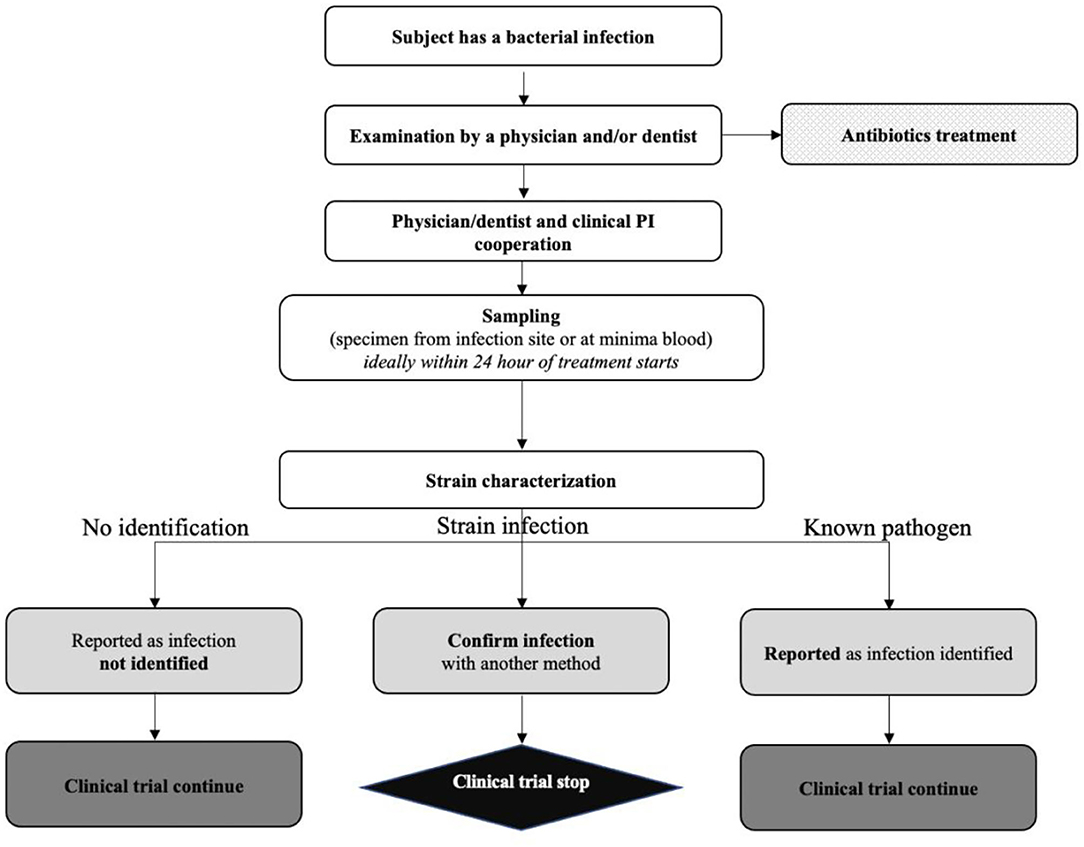
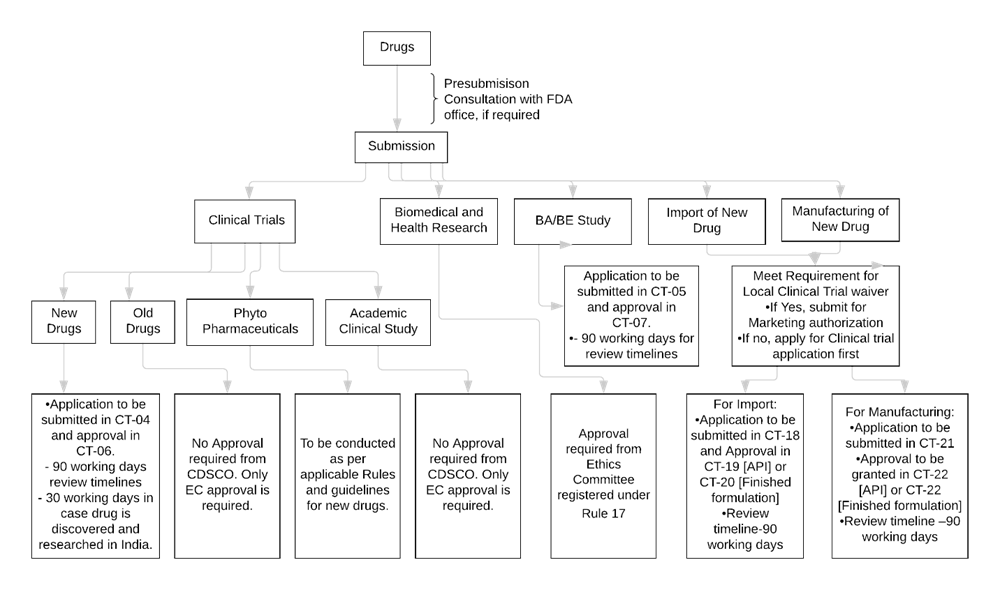
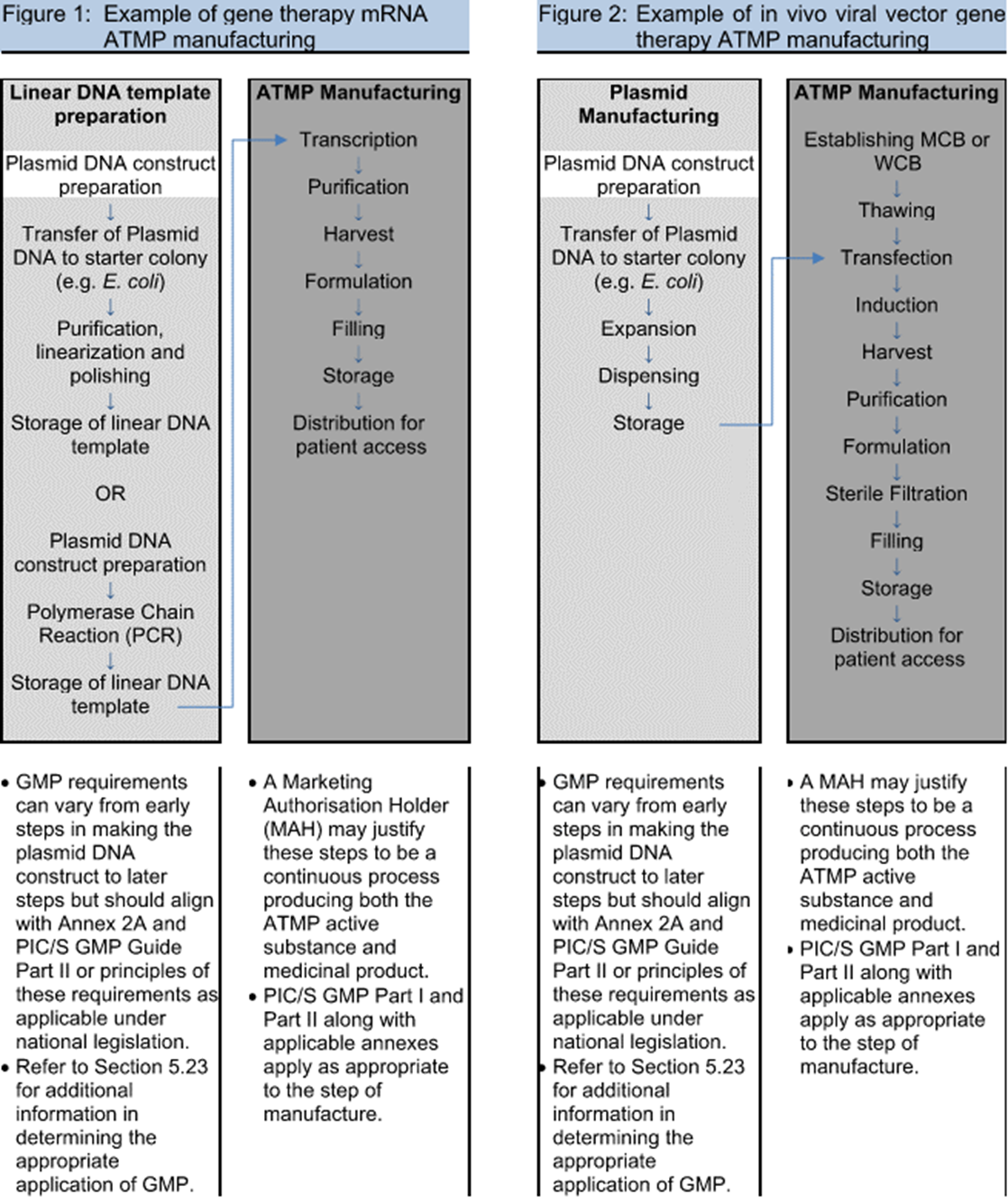
Clinical trials were missing from regulatory documents of extended-release methylphenidate for ADHD in adults: a case study of public documents - Journal of Clinical Epidemiology
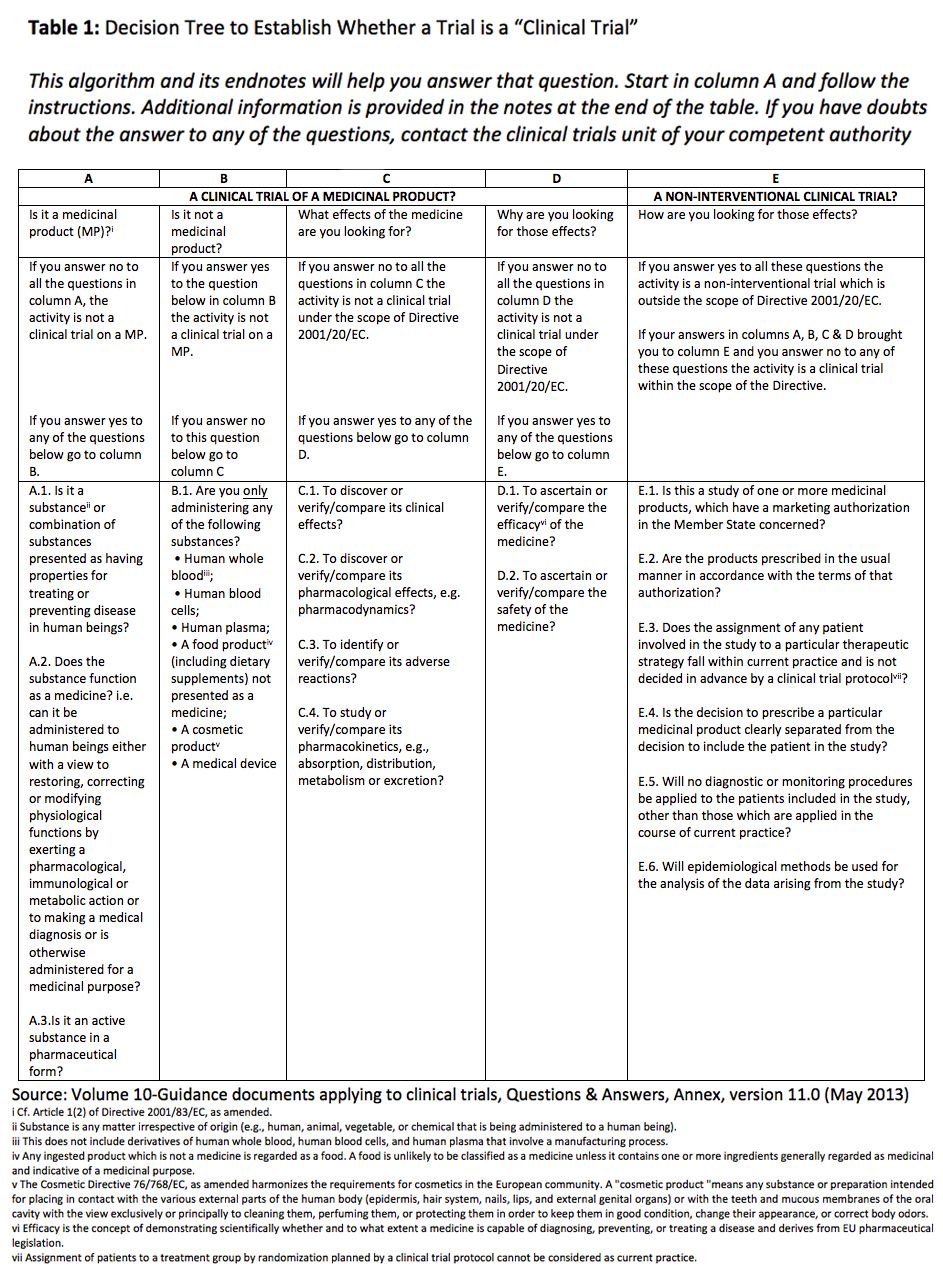
Interventional vs. Non-interventional Study Classification in the EU: Considerations on the Impact of Direct-to-Patient Contacts

Solve Expiry Labels, DtP, and Timelines for EU 536/2014 Clinical Trials Regulation | Healthcare Packaging
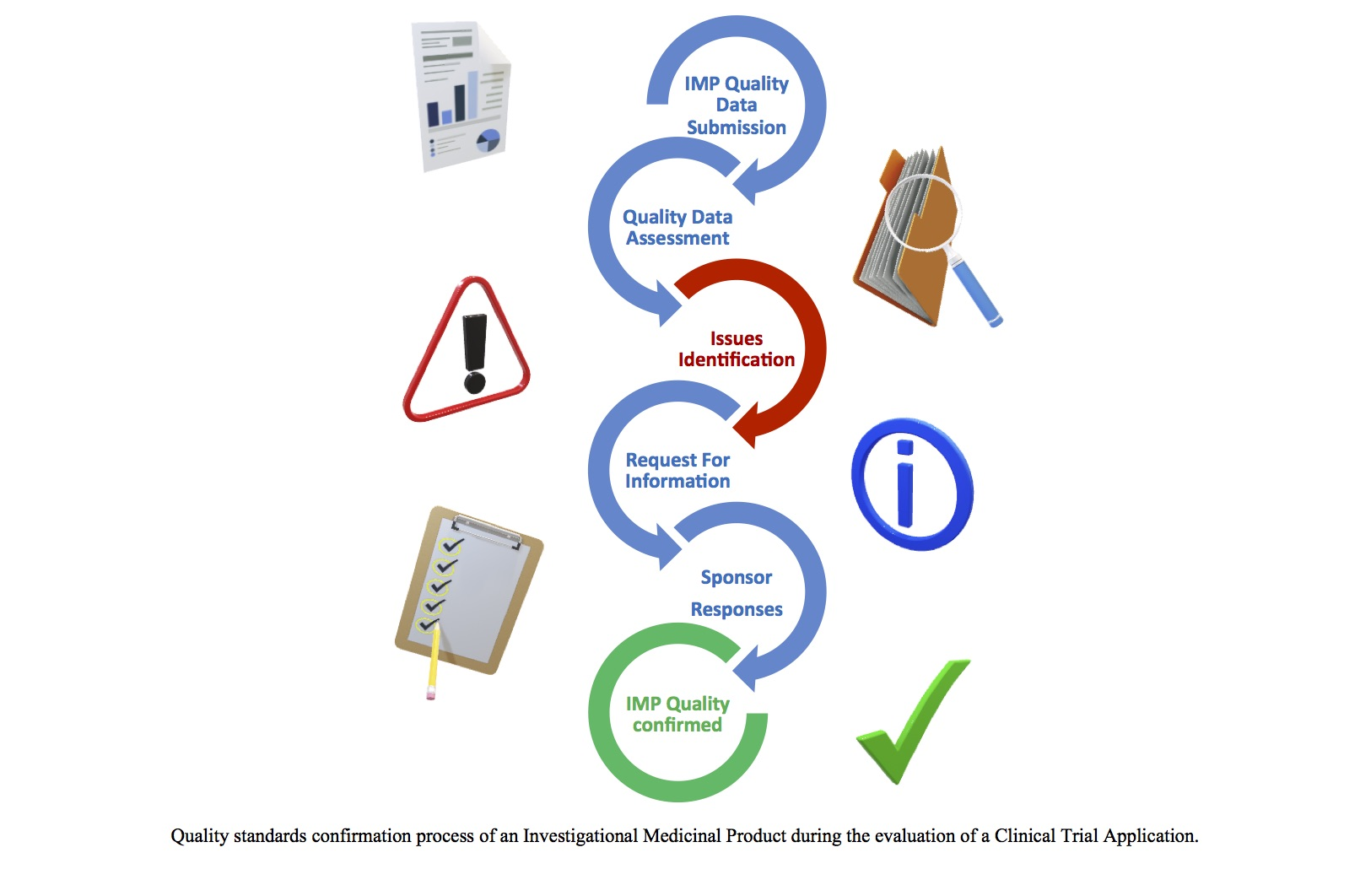
Pharmaceuticals | Free Full-Text | Quality Assessment of Investigational Medicinal Products in COVID-19 Clinical Trials: One Year of Activity at the Clinical Trials Office | HTML
CLINICAL TRIAL APPLICATION FORM African Vaccine Regulatory Forum (AVAREF) Clinical trial application form Trial's full title Sho

Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device - PDF Free Download
Annex 1: CLINICAL TRIAL APPLICATION FORM (CTA) To be completed by Applicants for all Clinical Trials Study Title: Protocol No:
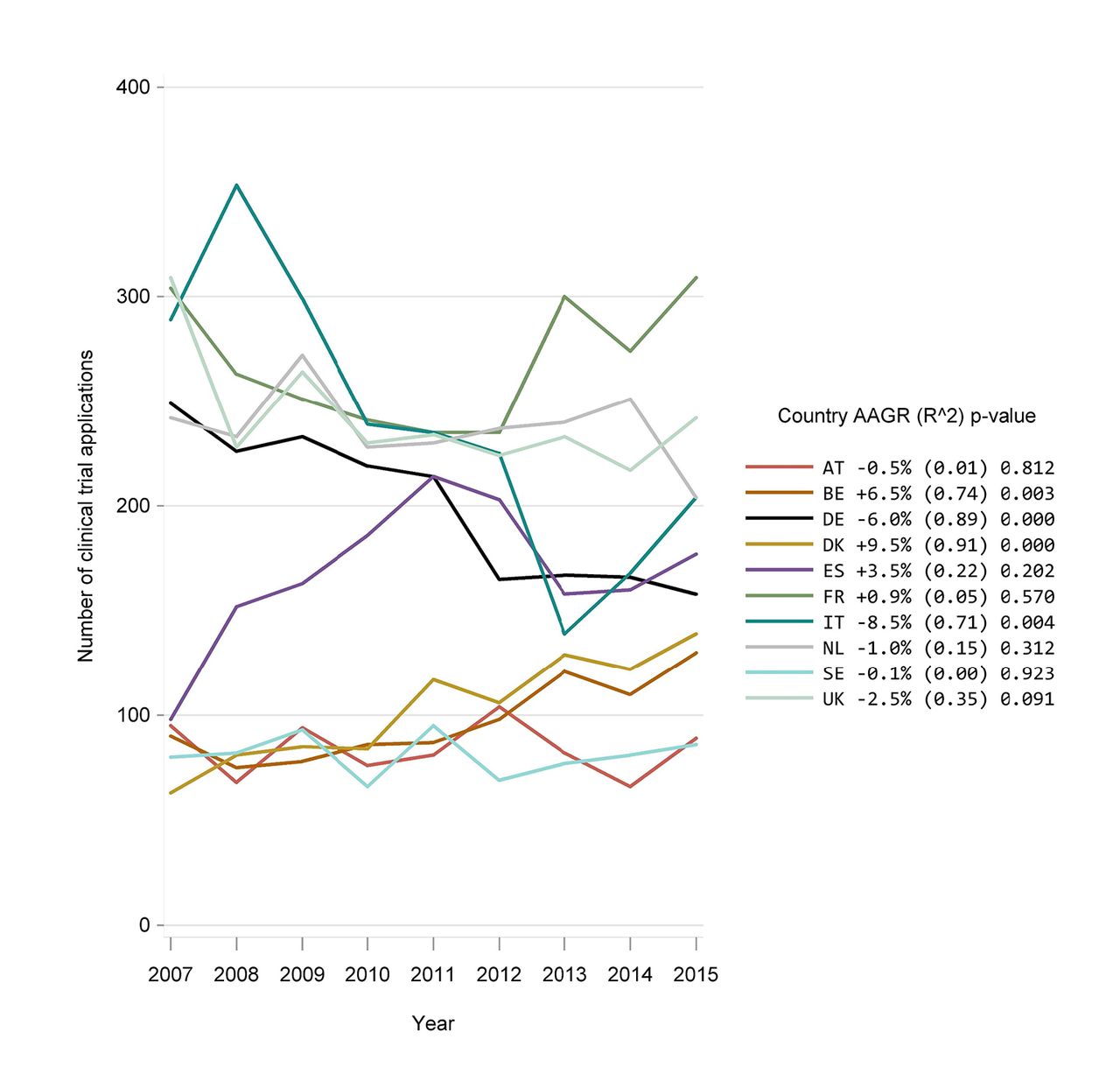
Development in the number of clinical trial applications in Western Europe from 2007 to 2015: retrospective study of data from national competent authorities | BMJ Open

Guidance Document: Part C, Division 5 of the Food and Drug Regulations “Drugs for Clinical Trials Involving Human Subjects” (GUI-0100) - Canada.ca